ALCOA is a framework or set of principles that ensures data integrity that was introduced by the FDA. As well as being essential for compliance reasons, the ALCOA principles are becoming increasingly important to GMP.
You can’t just have data, though. Instead, you need to have data and you need to ensure the integrity of that data. When you have this, you have data you can use and rely on.
The ALCOA principles ensure data integrity. They apply to the following types of GMP records:
- Electronically recorded – data recorded using equipment that ranges from simple machines through to complex and highly configurable computerized systems,
- Paper-based – a manual recording on paper of a manual observation or of an activity.
- Hybrid – where both paper-based and electronic records constitute the original record.
Data Integrity refers to the completeness, consistency, and accuracy of data. Complete, consistent, and accurate data should be attributable, legible, contemporaneously recorded, original or a true copy, and accurate [ALCOA].
- ATTRIBUTABLE: data must be recorded so that it can be linked to the unique individual who produced it. Every piece of data entered into the record must be capable of being traced back to the time it was taken and to the individual who entered it.
- LEGIBLE: data must be traceable, permanent, readable, and understandable by anyone reviewing the record. This is expanded to include any metadata pertaining to the record.
- CONTEMPORANEOUS: data are data that are summarily entered into the record at the time they are generated.
- ORIGINAL: data, or the source data, is the record medium in which the data was first recorded. An original data record should include the first data entered and all successive data entries required to fully detail the scope of the project.
- ACCURATE: data are correct, truthful, complete, valid, and reliable. Controls put in place to assure the accuracy of data should be implemented on a risk-based structure.
Data Integrity in Warning Letters
Alongside a rising number of warning letters from regulators that address the topic of data integrity, a whole series of new regulations were also published by the FDA, EMA, WHO, PIC/S and others. Many of these regulations are still at the draft stage, and they show that the concept of data integrity and its associated requirements are still in flux.
Based on ALCOA attributes being adhered to the data can be trusted, this becomes both simpler and more complicated when we introduce electronic systems capable of managing all these attributes as part of the ‘meta-data’ and have to consider how we can retrieve, store and archive data across it’s entire life cycle.
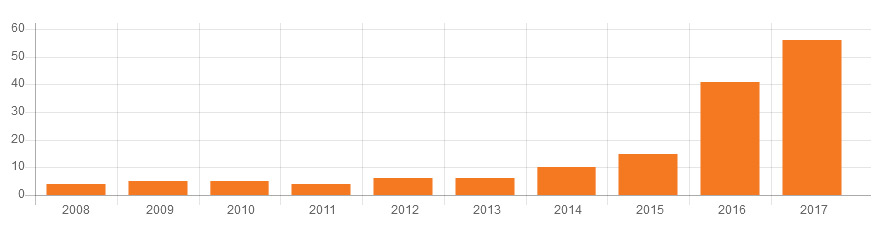
The Graphic below presents data integrity associated warning letters over the last 10 years, CY2008 through CY2017.
How Do You Meet Data Integrity Requirements?

Keep in mind that when basic data integrity controls are lacking, inspectors cannot rely on the data or records of that company determine compliance, quality or safety risks to patients.
We focus on your systems to ensure the integrity of data generated and maintained by your computerized systems in a regulated GxP environment.
- Are all data generated and collected Attributable to the person generating the data?
- Are all data recorded Legible and permanent?
- Are all results, measurements, or data Contemporaneous?
- Are all records Original ?
- Are all records and data Accurate ?

